Abstract
Background
Hospitalised patients with severe COVID-19 (requiring critical care level support) appear to be at increased risk of thrombosis despite standard pharmacological thromboprophylaxis. The magnitude of thrombotic risk in patients with COVID-19 of moderate severity (not requiring critical care) is less clear. The optimal approach to thromboprophylaxis (and the role of intensified thromboprophylaxis) remains to be determined.
Evidence of endothelial dysfunction has been widely reported in COVID-19 (particularly in severe COVID) and this may contribute to hypercoagulability.
Aim
To assess differences in patterns of hypercoagulability and endothelial dysfunction between a group of patients with moderate COVID-19 and a group of age-matched hospitalized patients (SARS-CoV-2 PCR negative) receiving low molecular weight heparin (LMWH) thromboprophylaxis.
Methods
Blood was collected from individuals admitted to hospital with COVID-19 of moderate severity (not requiring critical care level support) and a group of age-matched patients admitted with infective/inflammatory illness (SARS-CoV-2 PCR negative). All subjects received standard-dose LMWH thromboprophylaxis, with blood drawn at 12 hours post-dose (and with measurement of anti-FXa activity levels). Circulating levels of endothelial & fibrinolytic markers including ICAM, PAI-1, VCAM, soluble thrombomodulin (sTM), and tissue plasminogen activator (tPA) were determined by ELISA. Thrombin generation (TG) in platelet-poor plasma was assessed by calibrated automated thrombography in the presence of tissue factor (Final concentration, 1pM & 5pM), thrombomodulin (TM) (Final concentration, 6.25nM), and an inhibitory anti-tissue factor pathway inhibitor antibody (anti-TFPI; Final concentration 100μg/mL).
Results
14 COVID-19 positive subjects and 11 hospitalized controls were recruited. There were no differences in mean age (69.7±4.5 vs 61.6±4.7 years; p= 0.2) or mean Body mass index (25.7±1.1 vs 22.7±1.2 Kg/m2; p=0.1) between groups. No COVID-19 patient or control required critical care support. In the COVID group, radiological evidence of pneumonitis [diffuse (n=3) or peripheral infiltrates (n=7)] was present in the majority of cases. None of the COVID-19 cases were requiring supplemental oxygen at the time of recruitment.
All controls were admitted with either respiratory or urinary infection [radiological evidence of pneumonia in 4/11; supplemental oxygen requirement in 2/11, (28-36% FiO2 via nasal cannula)].
Plasma levels of sTM, ICAM, PAI-1 & VCAM were similar in both groups. Levels of t-PA were significantly higher in the COVID group (8.31±4.35 vs 4.91±2.37 ng/mL; p= 0.005). Despite similar plasma anti-Xa activity in both groups (0.06 vs 0.04 IU/mL; p=0.2), mean endogenous thrombin potential (ETP) was significantly higher in the COVID group (1929±119.7 vs 1528±138.9 nM*min; p=0.02), although peak thrombin was similar (173.6±26 vs 161.5±31nM). ETP-TM ratio was similar between groups (0.3±0.1 vs 0.2±0.1; p=0.3).
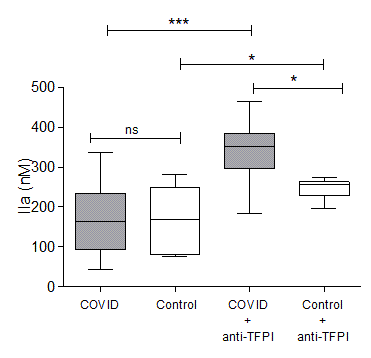
Despite increased ETP, the lag time to thrombin generation was significantly prolonged in the COVID group (8.3±0.6 vs 5.8±0.5 mins, p= 0.006). This pattern has previously been observed in vascular diseases associated with altered plasma tissue factor pathway inhibitor (TFPI) activity. In the presence of an anti-TFPI antibody, the difference in lagtime between groups was attenuated (4.7±0.2 vs 3.5±0.1 mins; p= 0.002) and the difference in overall thrombin generation (delta TG) between both groups became significantly increased (Fig.1).
Conclusion
Plasma thrombin generation is enhanced in patients with non-severe COVID-19 despite pharmacological thromboprophylaxis. Endothelial dysfunction is also observed in this group and appears to modulate parameters of plasma thrombin generation. The clinical implications of these observations are not known although clinical studies of intensified thromboprophylaxis in attenuating thrombotic risk and other complications are ongoing.
Fig 1. Inhibition of TFPI activity enhances thrombin generation in COVID-19.
In the presence of an inhibitory anti-TFPI antibody, peak plasma thrombin generation was enhanced in COVID-19 in contrast to that observed among SARS-CoV-2 PCR negative hospitalised patients (339.6+25.2 vs 247.4+10.1, p=0.01).
Maguire: Actelion: Research Funding; Bayer Pharma: Research Funding. Ni Ainle: Daiichi-Sankyo: Research Funding; Actelion: Research Funding; Leo Pharma: Research Funding; Bayer Pharma: Research Funding. Kevane: Leo Pharma: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal